When considering FDA guidelines and other government-mandated safety regulations — as well as requirements dictated by medications themselves — creating attractive, safe, and practical pharmaceutical packaging designs can seem overwhelming. With advancements in both packaging and packaging manufacturing technology, pharmaceutical companies are faced with numerous options.
TYPES OF PHARMACEUTICAL PACKAGING
As a result of government reforms and increased price pressure from insurance companies, pharmaceutical developers and manufacturers are working hard to identify areas for cost reduction. Many companies are now making use of packaging automation systems to reduce cost and increase throughput.
During pharmaceutical product development, it’s critical to make use of packaging that will optimize the manufacturing processes and appeal to consumers. Three common types of packaging solutions include:
▪️ Secondary packaging of drug delivery devices and other pharmaceutical products
▪️ Various over-the-counter (OTC) packaging methods
▪️ Automation trays
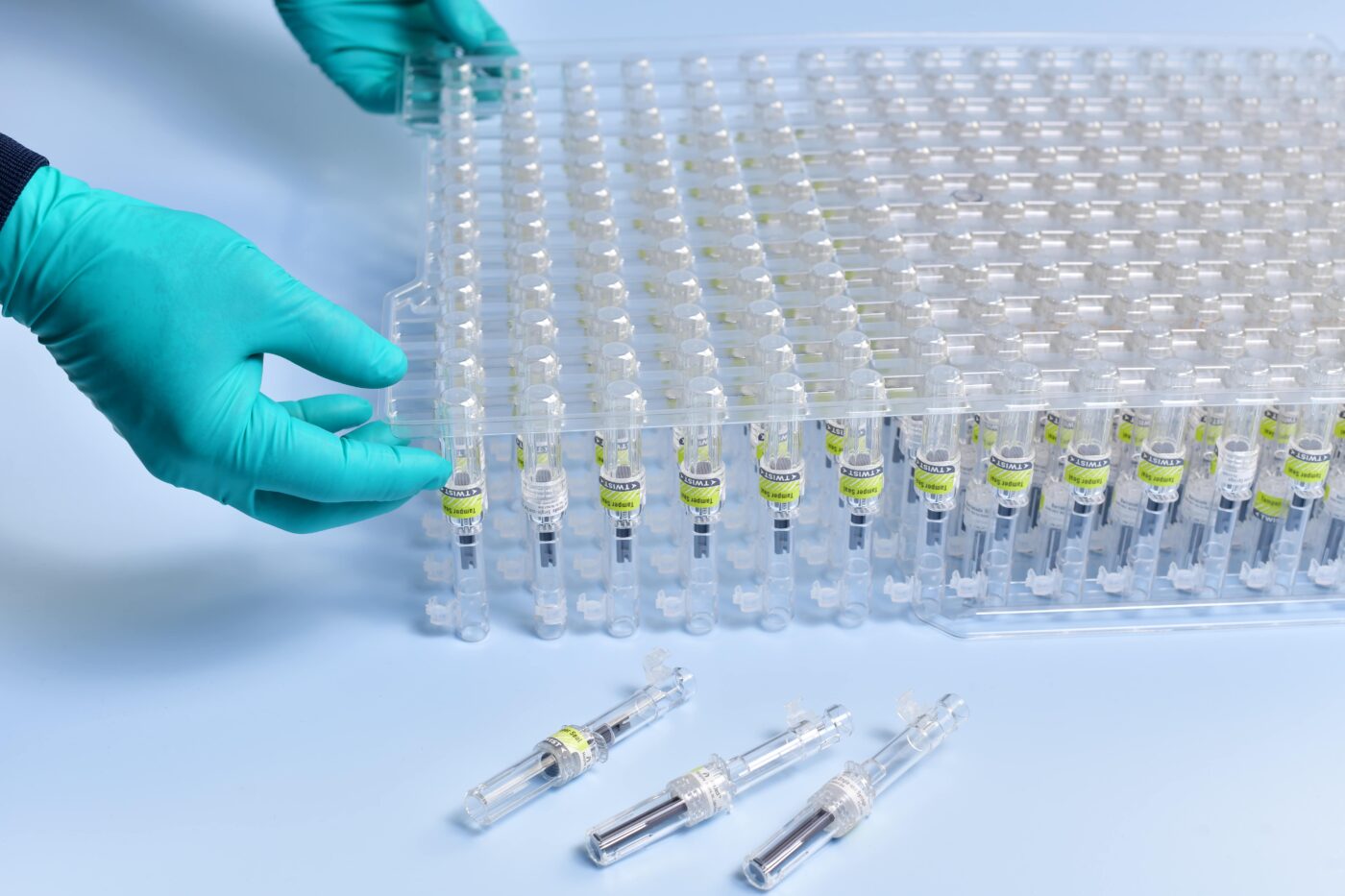
Secondary packaging is used to package together multiple delivery devices, such as syringes. In this scenario, the syringes themselves are the primary packaging, as they come into direct contact with medications.
OTC packaging solutions include branding-centric clamshell, blister, and tray packaging. OTC thermoformed packaging not only delivers a valuable brand experience, but it also ensures safe product delivery and security through the logistics cycle.
Automation trays — also known as Work in Process (WIP) trays — provide companies with a critical tool for the delivery of injection molded housings, completed devices, and sensitive components from one manufacturing location to another. These trays, when designed correctly, integrate seamlessly into contract manufacturers or pharmaceutical companies’ automated equipment. Today’s delivery devices contain sensitive printed features and are comprised of materials that may scratch or become damaged easily. WIP trays are critical in protecting these high-value components during the manufacturing and distribution cycle.
THE GOALS OF PHARMACEUTICAL PACKAGING
Pharmaceutical packaging is designed with several unique goals in mind, including:
▪️ Patient safety (always the primary goal)
▪️ Dose compliance
▪️ Ease of use
▪️ Product fit
▪️ Product integrity protection
▪️ Cost effectiveness
▪️ Transportation protection
▪️ Compliance with automation
▪️ Automation line integration and optimization
“Plastic Ingenuity is an innovative, super-motivated, friendly, and fun-to-work with team. They make things turn around in no time and have tons of ideas as solutions. They are vertically integrated, and hence it’s a one-stop shop for many [molds] and parts. The team goes the extra mile to work with other partners in a project and work seamlessly to accomplish a bigger objective.”
— Global Packaging Manager, GlaxoSmithKline
WHAT IS PACKAGING AUTOMATION?
Packaging automation is becoming increasingly important to the pharmaceutical industry. As the public’s pharmaceutical demand increases across the globe — not only in traditionally large markets such as the U.S., Western Europe, and Japan, but also in rapidly growing markets such as China, India, Turkey, Brazil, and Mexico — so too does the pharmaceutical industry’s demand for packaging.
To keep up with this demand, companies look for ways to increase production and packaging efficiency while keeping costs reasonable. Time and time again, they seek to automate their packaging processes as a solution.
Packaging automation, which often includes custom automation solutions, aims to automate the entirety of the packaging process. More than simply filling bottles, packaging automation includes automating every step of the process.
HOW PACKAGING AUTOMATION EQUIPMENT WORKS?
1. Remove the packages from the shipping boxes
2. Load the packages into the denester for separation
3. Denester places the separated packages onto the automated conveyor line
4. Robotics load the product and dose-compliant insert into packagin
5. Robotics label the filled package
6. Robotics seal the filled package
7. The packages are stored into shipping boxes
Automating the packaging process is not one size fits all — a specially engineered packaging solution is typically required. But the benefits of implementing an automated packing process, which include both increased production speed and efficiency, are well worth the effort.
When designed with a denesting strategy in mind, engineered packaging solutions maximize space during shipment. An example of a common space-saving method is when unfilled packaging products can be stacked together, each part fitting precisely over the others in a closely fitted stack. Stacking, or nesting, saves a tremendous amount of shipping space, allowing you to maximize volumes of packages while minimizing delivery costs. However, stacked packaging parts – especially small, thin, plastics common to the pharmaceutical industry – can be difficult to separate. This is where a denester comes in.
WHAT IS A DENESTER?
Part of any thoroughly designed custom automation solution, a denester is a highly specialized piece of equipment. Its job is to receive a stack of nested packages, separate (or denest) them into individual pieces, and feed those pieces into the packaging assembly line.
THE IMPORTANCE OF BRANDING
Custom packaging solutions not only help pharmaceutical companies meet unique delivery system requirements, but they also allow companies to brand their products in unique and eye-catching ways. Consumers won’t be as inclined to purchase a generic-looking product; well-designed, visually pleasing packaging will help increase sales and provide industry exposure.
This becomes even more critical as many industry experts foresee direct-to-consumer marketing becoming the norm, whether through internet ads and television commercials or the use of apps to access product information and related resources quickly and easily. With pressure to drive down the cost of drugs in today’s changing landscape, recognizable and attractive branding is paramount.
OVERCOMING DESIGN & MATERIAL SELECTION CHALLENGES
The implementation of any new system presents challenges. This is true almost universally, and implementing a custom automation solution into a pharmaceutical manufacturing environment is no exception.
The causes of these common challenges include material choices (such as PET and PETG), packing density choices, accommodation of existing or available nesting and denesting technologies, and sterility concerns. Luckily, issues can be easily remedied if they are considered early in the design process. It’s imperative to design the package up front, with the full system view, automation equipment, distribution environment, and consumer engagement and interaction considerations.
COMMON CHALLENGES
New engineered packaging solutions most commonly experience issues at the denesting phase. Interestingly, these issues arise from decisions made during the packaging design and manufacturing processes as opposed to those made during the design of the automation system itself. Proper draft and material choices play a major role in avoiding some of the common challenges.
What is Draft?
Draft is the angle of the sidewall of a part. A blister pack designed to hold 10 individual pills will have 40 different draft angles, four walls for each pill. A fully vertical sidewall has a draft of 0 degrees — this not only makes stacking more difficult, but also increases the chances that individual parts will stick to each other.
Maintaining a draft of at least 3 degrees will notably increase ease of stacking and notably decrease sticking between parts. Plastic Ingenuity maintains standard draft angles of 3, 5, 7, 10, 12, and 15 degrees, with 5 and 7 degrees being the most common. However, draft angle requirements change based on application. Plastic Ingenuity design engineers have experience determining the optimal balance between draft demands, equipment, and application.
What is the Difference between PET and PETG?
Commonly used for packaging such as trays and clamshells, PETG is a standard polyethylene terephthalate with the addition of a glycol modifier. The glycol modifier prevents crystallization, which causes PET to become brittle. Because PETG is much less susceptible to becoming brittle, it’s suitable for higher temperature processing procedures such as radio frequency (RF) sealing, heat seals, and sterilization. Selecting the correct plastic material for an application is also critical to the success of a packaging project.
MAXIMIZE EFFICIENCY DURING SHIPPING
When aiming to maximize efficiency during shipping, it’s likely that your first thought is to pack manufactured packaging parts as densely as possible — higher packing density means more parts shipped per container, which means lower shipping costs for you.
There is, however, a very real possibility of over packing, which will lead to denesting problems as well as production issues down the line. Packaging should be designed with a balance between packing density and ease of use in mind. Parts should be designed to be packed as densely as possible without negatively affecting denesting at your processing facility. A stack of parts that denest poorly will cause frequent jams on automation lines.
As discussed above, draft is an important design consideration that, when done properly, can increase possible packing density while also easing denesting. Inclusion of stacking lugs in the design of the packaging can prevent denesting issues when other methods are not an option.
“Plastic Ingenuity is our go-to supplier — especially if a short lead time is critical, because we can’t afford to do it again. Design work is a top strength for Plastic Ingenuity. They are very knowledgeable, have great software tools, they’re accessible, and they have knowledgeable and easy-to-work-with designers.”
— Supply Chain Manager, Nemera
ACCOMMODATING NESTING AND DENESTING EQUIPMENT
A packaging manufacturer may have existing nesting equipment on-site, used to stack and pack finished goods. This equipment will have a maximum number of parts that it can stack — say, 20, as an example. If the selected packing materials can accept stacks of 30 parts, it will be challenging to use the automated nesting system. In addition, if the pharmaceutical company has automated unpacking and denesting equipment with maximums of 25 parts, these problems will occur at the production end as well.
Further compounding these issues is the fact that some denesting equipment requires specific features, such as precisely spaced ribs and equipment tolerances, to properly denest a product; these features are sometimes overlooked during the design phase.
Plastic Ingenuity’s automation design engineers are often looped in as early as the design phase, so the package design is created with the automation equipment in mind. Understanding specific equipment tolerances are a key input into the direct design of the packaging.
TYPES OF DENESTING EQUIPMENT
There are four main types of denesting equipment. They all perform the same denesting function but in notably different ways:
1. Air Jet — Highly focused jets of air separate parts
2. Suction Cups — Vacuum powered suction cups pick up parts one by one
3. Automated Fingers — Robotic fingers pry each part away from the stack
4. Screw System — A screw wedges between parts, separating one from the stack with every revolution
“With packaging playing such a significant role in the commercial success of today’s pharmaceuticals, it makes tremendous sense to include CMOs and contract packagers in on product development as early as possible.”
— Packaging Trends, Pharmaceutical Manufacturing Magazine
ARE CUSTOM AUTOMATION SYSTEMS COST EFFECTIVE?
This is one of the most common inquiries we receive at Plastic Ingenuity. The idea that custom automation solutions are prohibitively expensive to design, and implement is a common misconception.
For scenarios with high production volumes or extended production timeframes, custom automation solutions easily deliver a favorable ROI. Unlike complicated workarounds to standard equipment — equipment that is readily available but ultimately mismatched to your needs — custom automation can lead to long-term cost savings. This allows for faster and more efficient production, a reduced number of required operators, and higher-quality, higher-accuracy products.
Our job does not end when the last delivery leaves our facility — we remain available to work with you in resolving any automation issues you encounter, including on-site support, to fine-tune and fully optimize your process.
Learn more about our custom automation solutions by scheduling a free consultation with one of our packaging experts.